ABOUT AppalTRUST
MISSION
To investigate the impact of Federal Drug Administration (FDA) Center for Tobacco Products regulatory priorities in rural Appalachia through collaboration, education, and pioneering regulatory scientific research.
OBJECTIVES
Facilitate and pioneer tobacco regulatory science research in Appalachian Kentucky.
Support and create new opportunities for innovative tobacco regulatory science and contribute to the national efforts to advance tobacco regulatory science through education and collaboration.
COMMUNITIES
OF FOCUS
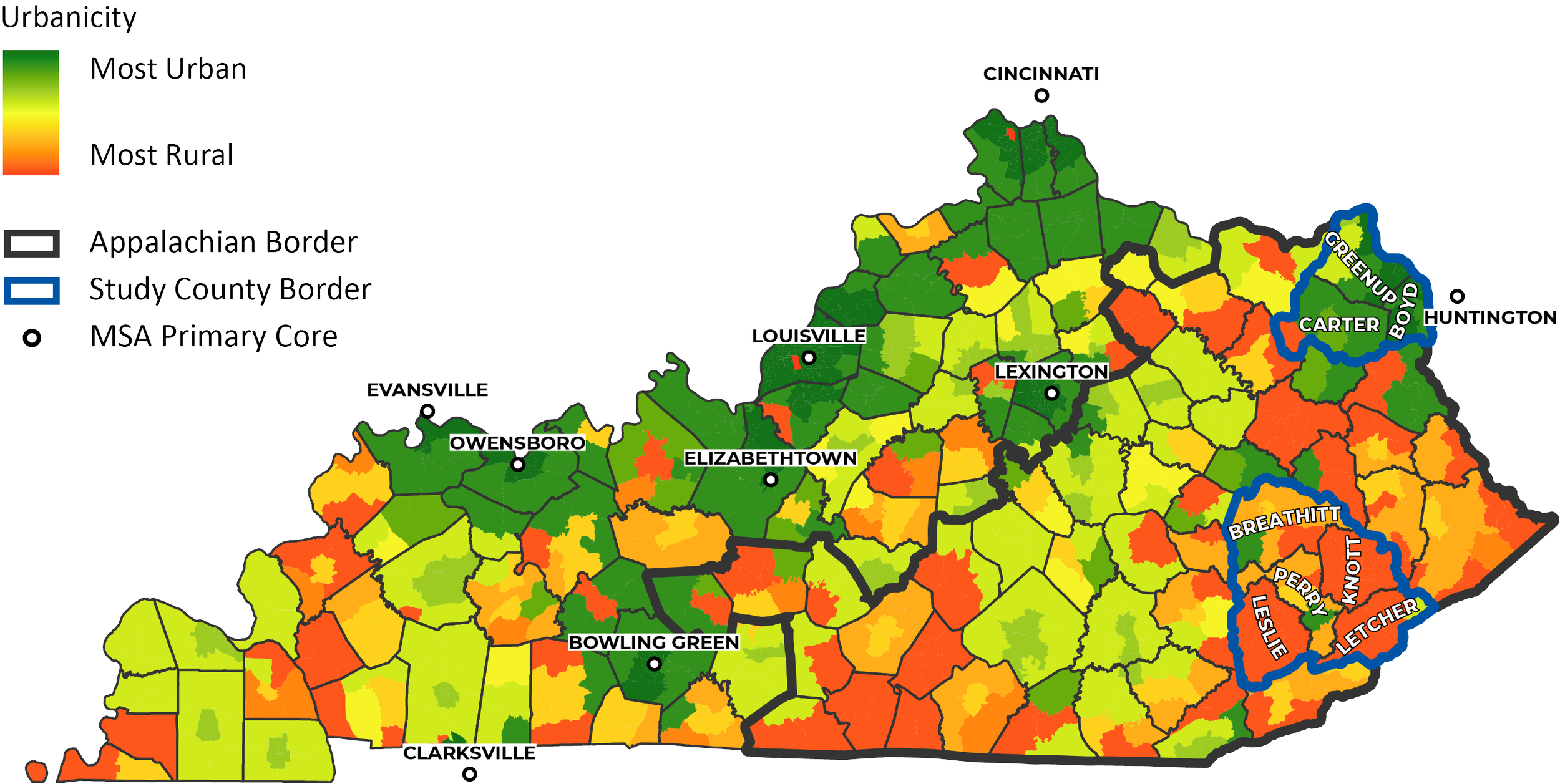
AppalTRUST research activities will focus on two groups of counties in Appalachian Kentucky:
AppalTRUST
RESEARCH
The FDA makes rules (called “regulations”) about which tobacco and nicotine products can be sold in the United States. The FDA how uses information from across the country to make those decisions. Before the AppalTRUST project, Appalachian voices were often not heard in the process of making those decisions.
The goal of the AppalTRUST Survey is to ensure that Appalachians have a say when the FDA makes decisions about tobacco and nicotine products. The AppalTRUST team will also gather information about where and how tobacco and nicotine products are marketed and sold in specific counties.
The team will use this information to examine how changes in FDA rules have an impact on the use and marketing of tobacco and nicotine products in Appalachian Kentucky. In particular, the team will compare the impacts across communities that are more rural and less rural (“relative rurality”).
Click here to learn more about the AppalTRUST education and training activities, faculty, staff, and scholars.